1st needle-free COVID-19 vaccines approved in China, India. What about Canada?
The next generation of COVID-19 vaccines is here – and it’s needle-free.
A nasal version of the COVID-19 vaccine has been approved in India, while regulators in China have also cleared an inhalable booster option administered through the mouth.
In Canada, all the approved COVID-19 vaccines are in the form of injections, but Canadian scientists are also developing a needleless technology that they believe could be more effective.
“This is really a novel and unique approach to vaccination,” said Dr. Fiona Smaill, professor of infectious and medical microbiology at McMaster University.
“The COVID pandemic has pushed a lot of researchers now to think about different ways of giving vaccines that in the long run may be more effective.”
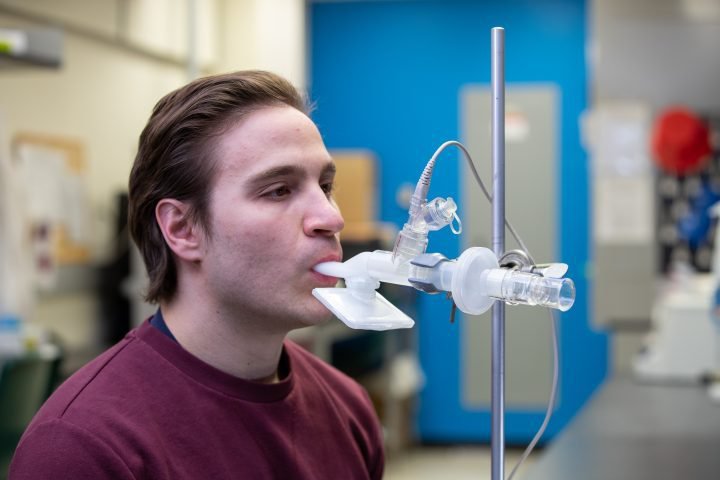
Smaill is leading the clinical trial, authorized by Health Canada, for two new adenovirus vector-based COVID-19 vaccines that can be administered by inhalation.
Unlike currently available vaccines that are injected, these are delivered by inhaled aerosol and target the lungs and upper airways, where respiratory infections begin.
While still in the early phases of development, with Phase I clinical trials ongoing, Smaill says this needleless technology could be a “game-changer” in terms of how we approach vaccination.
For a respiratory virus like COVID-19, generating an immune response in the lung with the inhaled vaccine is “much more effective” than in the bloodstream as is the case with an intramuscular injection, she explained.
The current Phase 1 clinical trial includes 36 healthy adults who have already received a pair of mRNA COVID-19 vaccine doses, such as Pfizer-BioNTech or Moderna, according to the university scientists.
“We’re looking at this as a booster to the currently available vaccine,” said Smaill.
These Canadian-made vaccines are among nearly a dozen possible shot-free candidates that are in various stages of testing globally, according to the World Health Organization (WHO).
Health Canada has also authorized a clinical trial for a single-dose intranasal vaccine developed by Altimmune, Inc., a U.S. biopharmaceutical company.
To date, the agency has not received a submission for market authorization of an inhaled COVID-19 vaccine.
“Should the department receive a submission for an inhaled COVID-19 vaccine, its review will be expedited,” said Anne Genier, a spokesperson for Health Canada.
The agency will also continue to provide regulatory advice to manufacturers developing “promising new products,” she added in a statement to Global News.
Needle-free versions are being explored as a strategy to improve protection against infection – and experts say they may be easier to administer than traditional vaccines.
On Tuesday, regulators in India authorized Bharat Biotech’s nasal vaccine as an option for people who haven’t yet been vaccinated.
CanSino Biologics announced Sunday that Chinese regulators have approved for emergency use an inhaled version of the company’s injected COVID-19 vaccine to be used as a booster dose.
The company pointed to preliminary results of studies suggesting the inhaled version revved up immune protection after one puff.
Smaill said it was “exciting” to see this next generation of vaccines moving quickly to the mainstream market.
The team at McMaster is currently looking for funding for Phase 2 of clinical trials of their vaccines and is aiming to include 500 participants.
As for when Canadians could possibly start receiving inhaled COVID-19 vaccines?
“We are still looking probably at least a couple of years down the road before we could embark on this as a clinical option,” said Smaill.
— With files from The Associated Press
by Global News







